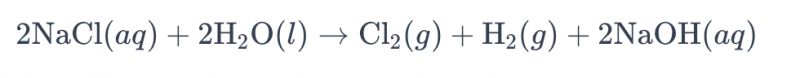Inqubo ye-electrolyzing isixazululo se-brine isebenzisa i-titanium electrode ukukhiqiza i-chlorine ivame ukubizwa ngokuthi "i-electrolysis of brine." Kule nqubo, ama-electrode e-titanium asetshenziswa ukuze kube lula ukusabela kwe-oxidation ye-chloride ion ku-brine, okuholela ekukhiqizeni igesi ye-chlorine. Isibalo samakhemikhali sesisonke sokusabela imi kanje:
Kule zibalo, ama-ion e-chloride athola i-oxidation ku-anode, okuholela ekukhiqizweni kwegesi ye-chlorine, kuyilapho ama-molecule amanzi encishiswa ku-cathode, akhiphe igesi ye-hydrogen. Ukwengeza, ama-ion e-hydroxide ayancipha ku-anode, enze igesi ye-hydrogen ne-sodium hydroxide.
Ukukhethwa kwama-electrode e-titanium kungenxa yokumelana nokugqwala okuhle kakhulu kwe-titanium, okuyivumela ukuthi iphendule ngokuzinzile ngesikhathi sokwenziwa kwe-electrolysis ngaphandle kokugqwala. Lokhu kwenza ama-electrode e-titanium abe yisinqumo esihle se-electrolysis of brine.
I-electrolysis yamanzi anosawoti ngokuvamile idinga umthombo wamandla wangaphandle ukuze unikeze amandla ngokusabela kwe-electrolytic. Lo mthombo wamandla ngokuvamile uwukuphakela kwamandla wamanje (DC) oqondile ngenxa yokuthi ukusabela kwe-electrolytic kudinga inkomba engaguquki yokugeleza kwamanje, futhi ukunikezwa kwamandla kwe-DC kungaletha inkombandlela yamanje engashintshi.
Enqubweni yokufakwa kwe-electrolyzing amanzi anosawoti ukuze kukhiqizwe igesi ye-chlorine, kusetshenziswa ugesi we-DC one-voltage ephansi. Amandla kagesi okunikezwa kwamandla ancike ezimeni ezithile zokusabela kanye nokwakhiwa kwemishini, kodwa ngokuvamile ahluka phakathi kwama-volts angu-2 kuya kwangu-4. Ukwengeza, ukushuba kwamanje kokuphakelwa kukagesi kuyipharamitha ebalulekile edinga ukunqunywa ngokusekelwe kusayizi wegumbi lokusabela kanye nesivuno sokukhiqiza esifunekayo.
Kafushane, ukukhetha kokunikezwa kwamandla kwe-electrolysis yamanzi anosawoti ancike ezidingweni ezithile zokuhlola noma izinqubo zezimboni ukuze kuqinisekiswe ukusabela okuphumelelayo kanye nokutholwa kwemikhiqizo efunekayo.
Isikhathi sokuthumela: Jan-16-2024